Abstract
Background Proliferating tumor cells are dependent on nucleotide synthesis to support DNA replication and RNA synthesis. The homologous CTP synthetases, CTPS1 and CTPS2, play a central role by catalyzing the rate-limiting conversion of UTP to CTP. Interestingly, while most tissues rely on CTPS2, lymphoid cells depend on CTPS1, as observed in rare patients with inherited CTPS1 loss of function manifesting selective B/T cell defects but no non-hematopoietic phenotype (Martin 2014, PMID 24870241). Herein, we investigate inhibition of CTPS1, alone or in combination, as a potential novel therapeutic target in mantle cell lymphoma (MCL).
Methods STP938 (Step Pharma) is a highly selective small molecule inhibitor of CTPS1 with >1,300-fold selectivity over CTPS2. In vitro cell proliferation was assessed at 72 hours by CellTiter-Glo. Synergy was quantified by Bliss scores. 3'seq-RNA profiling was performed before and after a 24-hour exposure to STP938; normalized data were subjected to KEGG pathway enrichment analysis. In vivo efficacy was established following subcutaneous transplantation of MCL cells (Z138) into immunodeficient mice (NSG, n=5 per treatment group); treatment was initiated when tumors reached approximately 50 mm3. STP938 was dosed at 30 mg/kg/day subcutaneously days 1-4 of a 7 day cycle; venetoclax at 75 mg/kg/day orally days 2-5 of a 7 day cycle for three cycles. Tumor size was assessed by calipers.
Results Analysis of the Achilles CRISPR dataset identified a dependency of human B cell lymphoma cell lines (n=34) on the de novo but not the salvage pyrimidine synthesis pathway. Of note, whilst CRISPR deletion of CTPS1 resulted in a marked proliferation defect, CTPS2 loss had no effect, consistent with the central role for CTPS1 in lymphoid cells established in human genetic studies. Analysis of publicly available data from primary MCL samples (n=122, GSE93291) identified high CTPS1 expression as a predictor of inferior overall survival (P=<0.0001).
A selective CTPS1 inhibitor, STP938, was used to evaluate a potential therapeutic role for CTPS1 inhibition. Of 15 MCL cell lines tested, 6 (40%) were highly sensitive to STP938 (IC50 <0.1 μM), 5 (33%) showed intermediate sensitivity (IC50 0.1 - 1 μM) and 4 (27%) were resistant (IC50 >1 μM). Of note, sensitivity to STP938 was independent of known indicators of poor prognosis, such as TP53 mutation, and was independent of resistance to standard of care therapies such as ibrutinib or venetoclax.
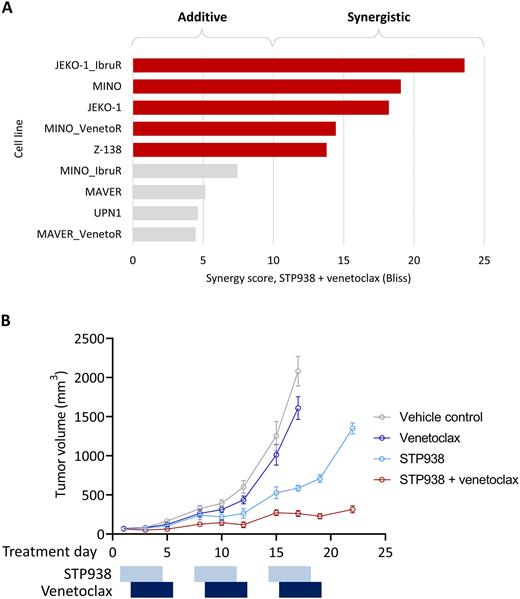
Pathway analysis of transcriptomic data (n=9 cell lines) identified downregulation of cell cycle pathways in response to STP938 exposure. Directed analysis identified modulation of BCL2-family transcripts, with STP938 inducing increases in BCL2 and BCL2L1 (BCL-XL) expression and a decrease in MCL1 expression (P=0.02, 0.006 and 0.02, respectively; paired t-test). BCL2 increase and MCL1 decrease upon CTPS1 inhibition have been further confirmed at the protein level (24h, 20nM). Given these findings, in vitro combination studies were undertaken with a selective BCL2 inhibitor. STP938 plus venetoclax demonstrated synergy in 5 of 9 MCL cell lines tested (Figure A). Of note, there was no association between synergy and sensitivity to single agent STP938, with two cell lines resistant to single agent STP938 (IC50 3.1 and >10 μM) nonetheless showing synergy with venetoclax.
The synergy between STP938 and venetoclax was further investigated in an in vivo model of MCL. Combination therapy was well tolerated. Single agent venetoclax showed no significant inhibition of tumor growth. Single agent STP938 slowed tumor growth to approximately 50% of vehicle control. The combination of STP938 and venetoclax showed strong synergy with more than 80% inhibition of tumor growth for 3 cycles of treatment (Figure B).
Conclusions Dependence of tumor cells on elevated rates of nucleotide synthesis has been exploited by agents such as cytarabine. Whilst therapy for hematological cancers is moving away from cytotoxic drugs in favor of targeted agents, inhibition of nucleotide synthesis remains an attractive target. STP938, a highly selective CTPS1 inhibitor, demonstrates single agent activity in vitro and in vivo, and shows strong synergy with the approved BCL2 inhibitor venetoclax. STP938 will enter clinical development for relapsed refractory B and T cell lymphomas in 2022.
Disclosures
Chiron:Step Pharma: Research Funding. Kervoelen:Step Pharma: Research Funding. Asnagli:Step Pharma: Current Employment. Parker:Step Pharma: Current Employment. Beer:Step Pharma: Current Employment. Pellat-Deceunynck:Step Pharma: Research Funding.
OffLabel Disclosure:
STP938 (Step Pharma) is a highly selective small molecule inhibitor of CTPS1 with >1,300-fold selectivity over CTPS2
Author notes
Asterisk with author names denotes non-ASH members.